Biological Safety Evaluation
Design & Engineering
Mechanical Design
Rapid Prototyping
Reverse Engineering
FEM Simulation
Quality and Validation
Quality System Support
VMP - DQ IQ OQ PQ Validation
Standard Operative Procedures (SOP) (IOP)
Technical File / Design Dossier
Design Control
DHF Design History File
Risk Management Process
Clinical Evaluation
Testing
Mechanical Testing
Analysis on Plastics
Biological Safety Evaluation
We perform tests, characterization and evaluation of biological safety of medical devices and related manufacturing processes:
• Using smart strategies instead of head down approach test programs with high associated costs
• In relation to specific processes and product, considering all available information relating to materials, products and similar processes
• In accordance with the directives, regulation and guidelines
• Defining methods that based on our experience extend far beyond the simple interpretation of the guidelines and the specific regulations
• With the final goal to ensure conformity of the product and patient safety

Our main areas of expertise are:
• Qualification of the biocompatibility of the devices / materials
• Qualification of procedures for cleaning, packaging, and sterilization
• Qualification and monitoring of production and packaging systems
• Validation of reusability

In particular:
• Selection of the approach of testing in a product-based or and / or process-based
• biological and chemical characterization of the profile of solubility, organic and inorganic leachable and extractable carcinogenic substances / impurities of materials (cytotoxicity tests, chemical analysis GC-MS, GC-FID, ICP-MS, et. Al.) (ISO 10993-1, -3 - 5, -7, -10, -11, -12, -13, -14, -18)
• Stability to corrosion of metallic materials, electrochemical test, immersion (ISO 10993-1, -15)
• hemocompatibility (ISO 10993-1, -4)
• biostability of the installations (ISO 10993-1, -6, -13, -15, -18)
• Overall assessment of the biocompatibility of toxicological risk management (ISO 10993-1, -17), in consideration of the characteristics and toxicological profile of the materials used, the substances released, literature data and experience with the application
• additional tests of biocompatibility after storage, reprocessing
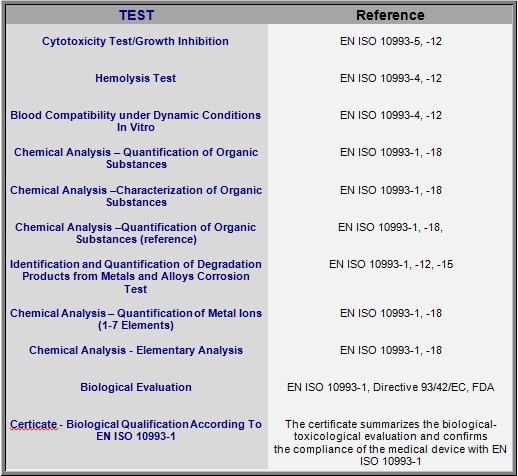
We carry out, in accordance with ISO 10993, the following tests to ensure the biological safety and biocompatibility of the analyzed product:
For any information relating to testing and / or monitoring of the biological safety of a medical device or its production process please do not hesitate to contact us!